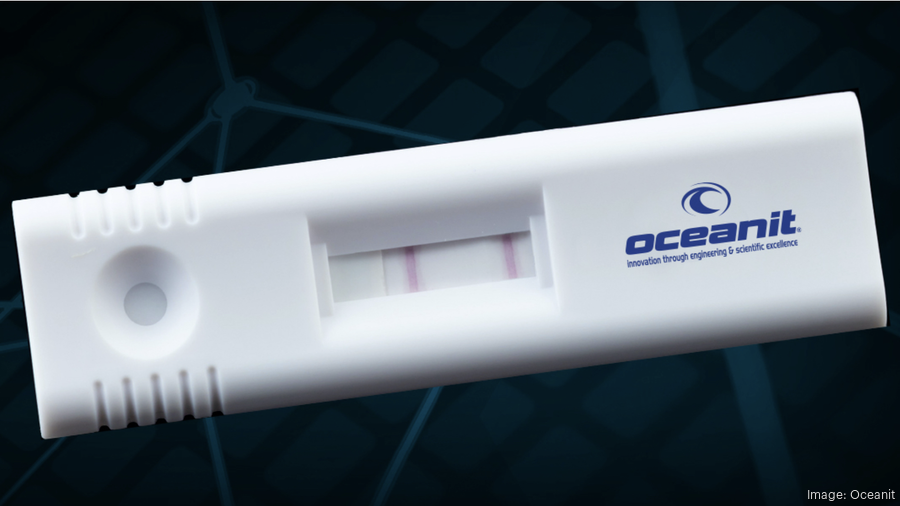
As Oceanit’s ASSURE-19 Covid-19 test makes its way through FDA trials, the test was also selected as a semifinalist for the XPRIZE for rapid Covid testing.
Oceanit was chosen from a pool of nearly 700 teams from around the world, and is the only Hawaii company moving forward. According to the competition's website, the winning teams will develop Covid-19 tests that are "radically affordable compared to what’s currently available on the market."
The XPRIZE Rapid Covid Testing competition is a $5 million dollar, 6-month competition to develop faster, cheaper, and simpler Covid-19 testing methods at scale. The goal of the competition is to remove barriers that can keep ideas and prototypes from hitting the ground running.
The XPRIZE Foundation is an international nonprofit, formed in 1994, that has funded innovative competitions in the domain areas of space, oceans, learning, health, energy, environment, transportation and robotics.
Oceanit said it entered this XPRIZE competition because "we too believe in the critical mission of affordable, regular testing: high-risk and already exposed persons should receive Covid-19 tests immediately and get results immediately."
In the coming phase, Oceanit will perform blind proficiency testing on up to 200 inactive virus samples sent to the company by the XPRIZE Foundation. The results of this blind testing will determine if the company reaches the final round of independent lab testing exercises.
Oceanit’s testing at The Queen’s Medical Center and with the University of Hawaii John A. Burns School of Medicine continues, having seen "great progress" to date, the company said. Oceanit's goal is to meet and surpass the 95% sensitivity and 95% sensitivity bars and capture enough data for FDA Emergency Use Authorization.
In PBN's recent innovation roundtable, CEO Patrick Sullivan told PBN that "early clinical work with Queen's looks excellent." Sullivan said he hopes to get FDA approval within three-to-four weeks.